
Insights+: The US FDA New Drug Approvals in November 2023
Shots:
-
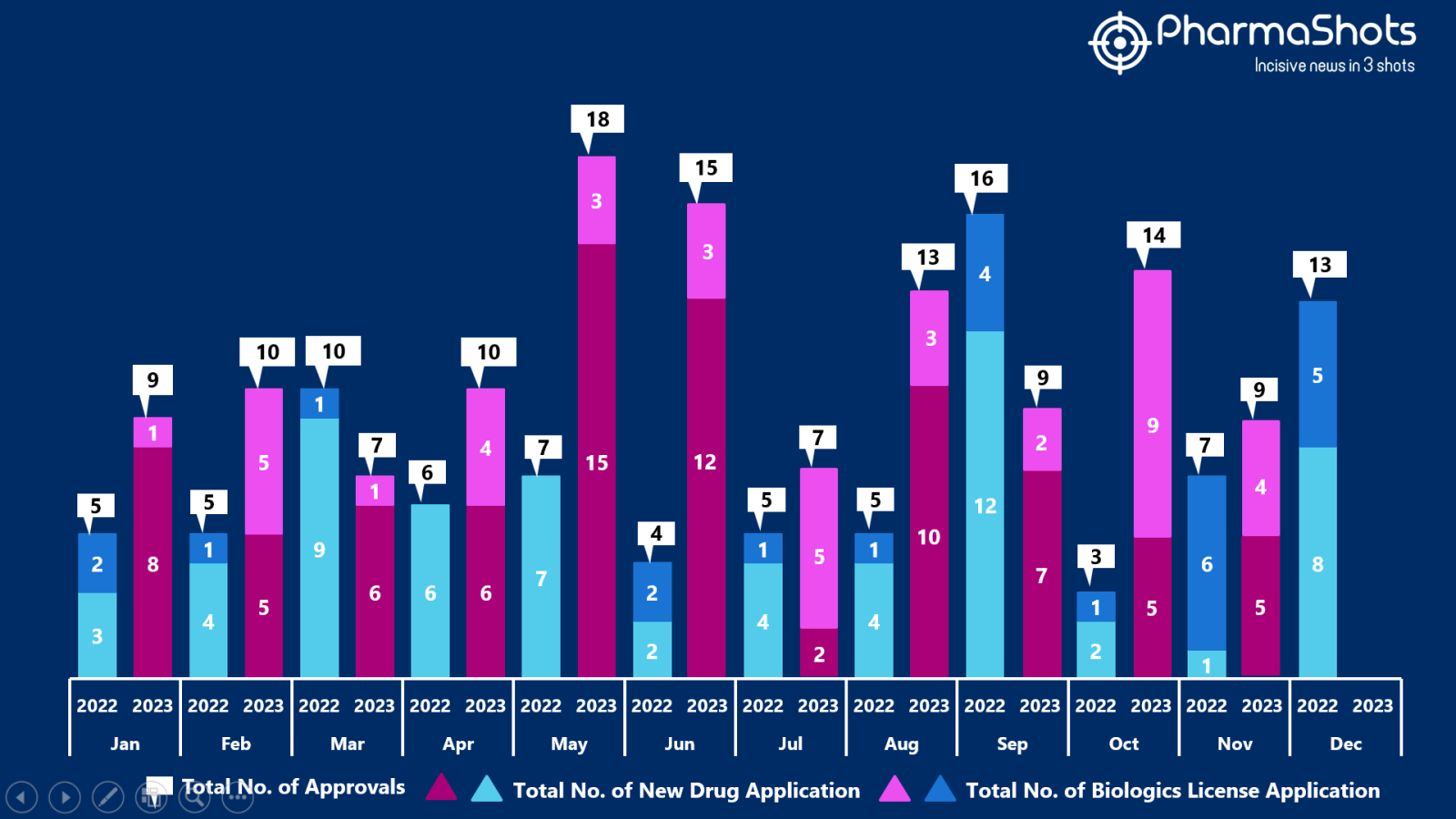
The US FDA approved 5 NDAs & 4 BLAs in November 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 109 novel products in 2023
-
In November 2023, the major highlights drugs were Wezlana (ustekinumab) approved for Multiple Inflammatory Diseases, and Cosentyx (secukinumab) for the Treatment of Hidradenitis Suppurativa
-
PharmaShots has compiled a list of a total of 8 new drugs, 1 biosimilar and 2 diagnostic tests are approved by the US FDA in November 2023
Wezlana
Active ingredient: ustekinumab Approved: Nov 1, 2023
Company: Amgen Disease: Multiple Inflammatory Diseases
- The approval was granted based on scientific evidence showing high similarity to Stelara with no clinically meaningful differences in safety and effectiveness and met the criteria to be interchangeable with Stelara
- Wezlana has been approved for the adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderately to severely active Crohn's disease and moderately to severely active ulcerative colitis
- It has also been approved for pediatric patients aged 6 yrs. to treat moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis
Novartis’s Cosentyx Received the US FDA’s Approval for the Treatment of Hidradenitis Suppurativa
Cosentyx
Active ingredient: Secukinumab Approved: Nov 1, 2023
Company: Novartis Disease: Hidradenitis Suppurativa
- The approval was based on 2 P-III studies (SUNSHINE) & (SUNRISE) evaluating the short (16wks.) & long-term (52wks.) efficacy, safety & tolerability of Cosentyx (IL-17A inhibitor, 300mg) vs PBO in 2 cohorts of patients (n=≥1,000) with moderate to severe HS. The 1EPs of both the study were HiSCR50
- The short-term results depicted that 44.5% & 38.3% of patients receiving doses at Q2W achieved HiSCR50 vs 29.4% & 26.1% whereas at Q4W the responses were seen in 41.3% & 42.5% vs 29.4% & 26.1%
- In addition, the 52wks. extended study of these patients showed an enhanced HiSCR50 response in both trials, with 65.0% & 62.2% responses in the (SUNRISE) trial and 56.4% & 56.3% responses in the (SUNSHINE) trial at dosing Q2W & Q4W
Phanthom Pharmaceutical Receives the US FDA Approval for Vaquenza (vonoprazan) for the Treatment of Erosive GERD
Vaquenza
Active ingredient: vonoprazan Approved: Nov 1, 2023
Company: Phanthom Disease: Erosive GERD
- The approval was based on a P-III (PHALCON-EE) study evaluating safety & efficacy of vonoprazan (10 mg) vs lansoprazole (30mg) in patients (n=1027) with erosive esophagitis
- The study met 1EP of complete healing by Week 8 with a 93% healing rate vs 85%. It demonstrated superior healing at Week 2 (70% vs. 53%). In the maintenance phase, voquezna surpassed lansoprazole (15mg) at 6 mos. for all patients (79% vs. 72%) & moderate-to-severe cases (75% vs. 61%)
- As a 2EP, it demonstrated non-inferiority in relieving heartburn over 6 mos. Vaquenza is an oral PCAB that inhibits stomach acid secretion. Vonoprazan, acquired by Phathom from Takeda, has demonstrated the potential to achieve crucial pH levels, enhancing its effectiveness
Keytruda
Active ingredient: pembrolizumab Approved: Nov 2, 2023
Company: Merck Disease: Biliary Tract Cancer
- The approval was based on the P-III (KEYNOTE-966) clinical trial evaluating Keytruda (200mg) + CT (gemcitabine, 1,000 mg/m2 & cisplatin, 25 mg/m2) vs PBO + CT, randomized in the ratio 1:1, in patients (n=1,069) with locally advanced unresectable/metastatic biliary tract cancer. In both cohorts, patients received Keytruda + CT or PBO + CT on Day 1 followed by CT alone on Day 8 for Q3W
- The results depicted OS to be 12.7mos. vs 10.9mos with mDOR of 8.3mos. vs. 6.8mos. along with a mPFS of 6.5mos. vs 5.6mos., ORR of 29% vs 29% with CR & PR of 2.1% vs 1.3% & 27% vs 27%
- The safety results of the study demonstrated a difference of ≥5% incidence in adverse reactions between patients in both cohorts for pyrexia (26% vs 20%), rash (21% vs 13%), pruritus (15% vs 10%) and hypothyroidism (9% vs 2.6%)
Alinity M High Risk (HR) HPV assay
Active ingredient: N/A Approved: Nov 3, 2023
Company: Abbott Disease: HPV Infections
- The Alinity m high-risk HPV assay has been approved for use as a test for HPV identification and for regular cervical cancer screening. Additionally, it is also approved in combination with a Pap test (co-testing)
- The assay is significant because it provides data on 5 risk groups encompassing the 14 distinct virus genotypes that may cause cancer
- The test will be available through the Alinity m laboratory instrument. Additional assays are available for use in the US incl. SARS-CoV-2, Resp-4-Plex, HCV, HBV, HIV-1, STI (CT/NG/TV/MG), CMV & EBV
Zepbound
Active ingredient: tirzepatide Approved: Nov 9, 2023
Company: Eli Lilly Disease: Obesity
- The approval was based on the results of 2 studies from the P-III (SURMOUNT) trials incl. (SURMOUNT-1) & (SURMOUNT-2) evaluating Zepbound (inj.) vs PBO in individuals (n=≥5,000) with obesity, excess weight & weight-related medical problems
- At 72 weeks, the patients (n=2,539) in the P-III (SURMOUNT-1) trial, showed a significant reduction in weight vs PBO. with individuals taking a low dose (5 mg) losing ~34lb & those on a high dose (15 mg) losing ~48 lb vs PBO (7lb). Additionally, compared to 1.5% in PBO, 1 in 3 patients receiving the highest dose, nearly lost 25% of their body weight
- Zepbound activates both GIP glucose-dependent insulinotropic polypeptide) & GLP-1 (glucagon-like peptide-1) hormone receptors. Zepbound is expected to be launched in the US by EY 2023 in doses 2.5, 5, 7.5, 10, 12.5, 15mg
Takeda’s Fruzaqla Receives the US FDA’s Approval for the Treatment of Metastatic Colorectal Cancer
Fruzaqla
Active ingredient: fruquintinib Approved: Nov 9, 2023
Company: Takeda Disease: Metastatic Colorectal Cancer
- The approval was based on the data from 2 P-III studies (FRESCO-2) & (FRESCO) trial evaluating the safety & efficacy of Fruzaqla vs PBO in patients (n=934 & 416) with previously treated mCRC. The approval was received prior to the planned PDUFA date of Nov 30, 2023, under priority review
- The results from the (FRESCO-2) & (FRESCO) trial depicted a mOS of 7.4mos. vs 4.8mos. & 9.3mos. vs 6.6mos. whereas mPFS seen in the (FRESCO) trial was 3.7mos. vs 1.8mos. The results from the trials were published in “The Lancet” & ”JAMA”
- Earlier the company received approvals for Fruzaqla, a selective oral VEGFR -1, -2 & -3 inhibitor, by the NMPA (Sep’18) & EMA (Jun’23) as a treatment option for mCRC & an application to the PMDA was submitted on Sep’23
Augtyro
Active ingredient: repotrectinib Approved: Nov 16, 2023
Company: BMS Disease: Non-Small Cell Lung Cancer
- The approval was based on P-I/II (TRIDENT-1) study evaluating the efficacy, safety, PK, & tolerability of Augtyro in TKI-naïve (n=71) and TKI-pretreated patients (n=56) with advanced solid tumors incl. NSCLC
- The 1EP of the study includes ORR & the 2EPs of the study include DOR, PFS & intracranial response in 6 distinct expansion cohorts incl. TKI-naïve and TKI-pretreated patients with ROS1+ locally advanced/metastatic NSCLC
- The result depicted that of the 79% of TKI-naïve & 38% of TKI-pretreated patients who responded to treatment, 6% & 5% depicted a CR whereas 73% & 32% experienced partial response along with an mDOR of 34.1 & 14.8mos. Moreover, patients with measurable CNS metastases showed responses in 7 of 8 TKI-naïve & 5 of 12 TKI-pretreated patients with intracranial lesions at baseline
DefenCath
Active ingredient: N/A Approved: Nov 16, 2023
Company: Cormedix Disease: Hemodialysis
- The approval was based on the P-III (LOCK-IT-100) study evaluating the safety & efficacy of DefenCath vs heparin (control) as a Catheter Lock Solution (CLS) to reduce CRBSIs in patients (n=806) with kidney failure receiving chronic hemodialysis through a central venous catheter (CVC)
- The result depicted a lower incidence of CRBSI in patients in the DefenCath cohort vs the control group with a Hazard Ratio of 0.29 thereby indicating a statistically significant reduction in risk of developing a CRBSI by 71%
- DefenCath is a CLS that helps prevent infection. It contains heparin & taurolidine, an antimicrobial agent designed to occupy the catheter lumen in between sessions of dialysis
Truqap
Active ingredient: ustekinumab Approved: Nov 20, 2023
Company: AstraZeneca Disease: Breast Cancer
- The approval was based on P-III (CAPItello-291) study evaluating efficacy of Truqap (400mg; BID) + fulvestrant vs PBO + fulvestrant on breast cancer patients (n=708). The Dual 1EP of the study was PFS on the overall population & on patients whose tumors have qualifying alterations in the PI3K/AKT/PTEN gene
- The result showed 50% reduction in death vs fulvestrant alone in patients with furthermore median PFS was 7.3 vs 3.1mos. The safety profile was consistent with previous data. The result was published in NEJM
- The US FDA granted Priority Review examined by Project Orbis under which Truqap + fulvestrant is under review by other regulatory authorities
Ogsiveo
Active ingredient: nirogacestat Approved: Nov 28, 2023
Company: SpringWorks Disease: Desmoid Tumors
- The approval was based on the P-III study (Defi) evaluating nirogacestat, vs PBO on Desmoid Tumor patients (n=142). The study met its 1EP depicted improved PFS & 71% reduction in disease progression
- Based on RECIST v1.1 ORR was 41% vs 8%; the complete response rate was 7% vs 0%; mDoR was 5.6 vs 11.1mos. Additionally, median PFS was not met in the nirogacestat arm & was 15.1mos. in PBO arm. Furthermore, Ogsiveo showed early & persistent benefits in patient-reported outcomes (PROs) such as pain, a symptom unique to desmoid tumors, role- & physical-functioning, & overall health-related quality of life
- The company anticipates submitting MAA for Ogsiveo, an oral, selective, small-molecule gamma-secretase inhibitor to the EMA in H1’24
Related Post: Insights+: The US FDA New Drug Approvals in October 2023

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.














